Cough, cough; Who’s there? Lungworm in cattle
In recent years, there has been a significant increase in the prevalence of lungworm, (also known as parasitic bronchitis/hoose/husk), in Irish cattle herds.
Dictyocaulus viviparus, the only species of parasite responsible for lungworm in cattle, can cause quite severe disease in animals even with low numbers. This can be attributed to the nature and extent of the incurable damage it induces in the animal’s airways. Therefore, an understanding of the life cycle of this parasite plays a pivotal role in the ‘prevention is better than cure’ rhetoric that underpins most modern animal-based agriculture and veterinary practices.
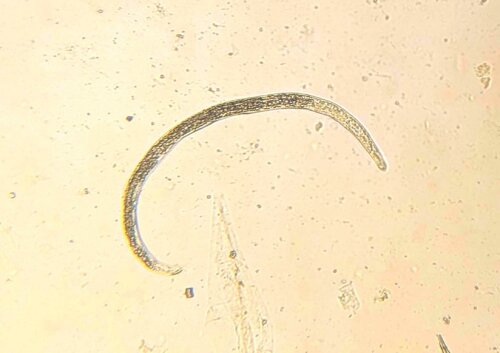
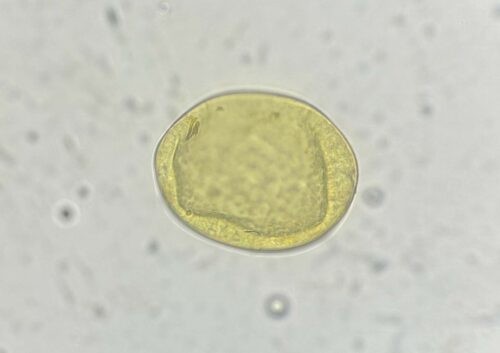
Adult parasites reside in the larger airways of cattle. Females lay eggs that immediately hatch into the first of several larval stages. The first, are coughed up by the animal, swallowed, pass through the animal’s digestive tract, and are eventually expelled in their faeces. These larvae do not feed to get energy, relying instead on storage granules of starch, similar to fat stores in the human body, that allow them to move around and get to where they want to be. Interestingly, one of these desired destinations is on top of the stalks of Pilobolus, a fungus that grows in cowpats, that harbours a rounded, spore-filled ‘sporangium’ on the top of its stalk that bursts once the fungus has reached maturity, dispersing spores (and subsequently lungworm larvae) up to several metres around it. This is an important step in the lungworm life cycle as cattle do not generally graze too close to their dung, so the fungus allows the larvae to move rapidly away onto cleaner areas of pasture where they are more likely to be ingested. The larvae develop through the summer months in temperate climates providing the most favourable conditions for them to become infective. At this stage they are eaten by the cow and transform in the digestive tract and then migrate to the lungs, where they finally mature into adults. The time taken from ingestion to these larvae becoming adults and producing eggs of their own is only 28 days.
It is at the stage where the larvae migrate to the lungs where the destruction happens, as the lungworm detrow the tissue as they migrate. Eggs of female adults drop into the lower airways causing ‘aspiration pneumonia (inflammation of lung tissue caused by foreign material getting trapped in the smallest air sacs or alveoli), blockage of the larger airways with high numbers of adult worms or ruptured alveoli that cannot be repaired by the body severely affecting the lungs from functioning well.
Clinical signs include:
- Lethargy.
- Coughing.
- Increased respiratory rate (i.e. more breaths per minute).
- Lack of appetite.
- Green-tinged nasal discharge.
- Standing with outstretched necks as if being starved of air (“air hunger”).
Affected animals are more susceptible to getting another bacterial infection. Up to 30% of severely affected animals will never fully recover from infection.
For the animals that do recover from infection, strong protective immunity is established that prevents any subsequently ingested larvae from completing their life cycle. Though this generally means that clinical signs during these successive infections should be prevented, occasionally a ‘re-infection syndrome’ can occur where older, previously-infected animals are subjected to a very severe parasitic challenge and show signs of respiratory distress that relate to their own inflammatory and immune response rather than that of parasitic migration and replication. Because of their age, first-grazing-season calves are the most susceptible group of animals to be affected by Dictyocaulus viviparus but continued exposure to light levels of infection during each subsequent grazing season will allow them to maintain optimum immunity.
Administration of anthelmintics to calves in their first grazing season is considered a common method of lungworm control. However, it is important to note that some strategies can be considered ‘too efficient’ as they can prevent the degree of parasitic challenge required by the animals for adequate immunity to develop and can predispose these animals to ‘re-infection syndrome’ once they’re older. Vaccination is also an option consisting of irradiated
larvae that are given to calves orally, on two separate occasions, one month apart. These irradiated larvae are capable of beginning their migration path inside the animal and survive long enough to elicit an immune response but do not survive long enough to produce eggs or cause clinical disease. This vaccine should be given to calves before exposure to pasture challenge; ideally having them housed for two weeks after their second dose. Though this may be impractical for spring-born calves, some studies have suggested that on-pasture vaccination can be effective provided that the level of pasture contamination is not too high. If vaccination is going to be used, it must be continued on an annual basis just in case a small proportion of the larvae inside the vaccine do go on to complete their life cycle and release eggs into the environment. Where animals are infected with adult larvae, anthelmintics will work to kill them off but the dead worms and their products will remain in the respiratory tract, meaning that a stimulus for the animal’s immune system will remain in the animal for some time. Supplementary treatment with anti-inflammatory medication may need to be prescribed or administered by a vet to alleviate exacerbated clinical signs in these animals.
The occurrence of outbreaks of lungworm is difficult to predict for the following reasons:
- Only relatively small numbers of parasites are required to initiate disease
- Parasites are spread rapidly on pasture through either their symbiotic relationship with Pilobolus or by rainfall (Pilobolus is capable of spreading larvae across field boundaries)
- Larvae are capable of surviving colder weather conditions in winter by burying into the soil and re-emerging during the start of the warmer summer months
- Occasionally larvae can undergo a stalled maturation or ‘hypobiosis’ inside the animal, meaning that the development of the larvae into their final adult stage is put on pause for one of a variety of reasons, but resumes at a later date. Animals that harbour hypobiotic larvae can serve as a reservoir of infection for susceptible calves the following year.
Though respiratory disease in calves at pasture is highly suggestive of lungworm infection, diagnosis can be confirmed using the Baermann technique on faecal samples. This involves placing faeces in a funnel-shaped container that is covered in warm water and allowed to stand for an hour or two, forcing the larvae to move out of the faecal material towards the bottom of the funnel where they can be collected and identified. It is important to note, however, that the absence of larvae does not necessarily rule out lungworm infection in coughing animals, as clinical signs caused by migrating or adult parasites may occur prior to the production of eggs and subsequent L1 larvae. In this case, faecal examination will have to be repeated at a later date in order for a definitive diagnosis to be made. There are reports of flotation methods similar to those used with the Micron kit being used to recover lungworm larvae from faecal samples, so watch this space!